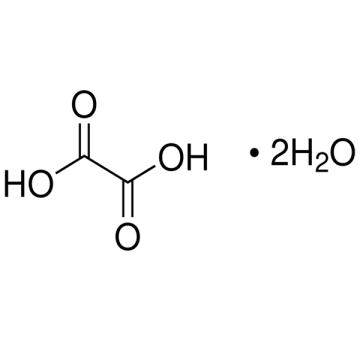
Sigma-Aldrich O0376 Oxalic acid dihydrate ReagentPlus®, ≥99.0% (GC) 3 kg
Kategori
Marka
Stok Kodu
LB.SA.O0376-3KG
Kısa Bilgi
Synonym(s): Ethanedioic acid dihydrate Linear Formula: HO2CCO2H · 2H2O CAS Number: 6153-56-6 Molecular Weight: 126.07 Beilstein: 3679436 EC Number: 205-634-3 MDL number: MFCD00149102 eCl@ss: 39021701 PubChem Substance ID: 57654475 NACRES: NA.21
Bilgi
CAS Number: 6153-56-6
| Sigma-Aldrich O0376 Oxalic acid dihydrate ReagentPlus®, ≥99.0% (GC) 3 kg |
| Synonym(s): Ethanedioic acid dihydrate Linear Formula: HO2CCO2H · 2H2O CAS Number: 6153-56-6 Molecular Weight: 126.07 Beilstein: 3679436 EC Number: 205-634-3 MDL number: MFCD00149102 eCl@ss: 39021701 PubChem Substance ID: 57654475 NACRES: NA.21 |

|
PROPERTIES
vapor density 4.4 (vs air)
Quality Level 200 vapor pressure <0.01 mmHg ( 20 °C) product line ReagentPlus® Assay ≥99.0% (GC) mp 104-106 °C (lit.) SMILES string [H]O[H].[H]O[H].OC(=O)C(O)=O InChI 1S/C2H2O4.2H2O/c3-1(4)2(5)6;;/h(H,3,4)(H,5,6);2*1H2 InChI key GEVPUGOOGXGPIO-UHFFFAOYSA-N DESCRIPTION General description Oxalic acid dihydrate (OAD) crystals are reported to be monoclinic with P21/n space group.[1] The electron density of OAD has been obtained using X-ray diffraction studies under high resolution.[2] Application Oxalic acid dihydrate may be used: - As a catalyst in the preparation of tetrahydroquinoline derivatives via imino Diels-Alder reaction.[3] - As a homogeneous catalyst in the preparation of highly functionalized piperidines via multi-component reaction.[4] - As an oxidant for the formation of triazolinediones from corresponding urazoles and bisurazoles via oxidation.[5] - In the transformation of thiols to nitrosothiols by reacting with sodium nitrite.[6] Legal Information ReagentPlus is a registered trademark of Merck KGaA, Darmstadt, Germany |
Tavsiye Ürünler
1.668,03 TL + KDV
2.001,63 TL
1.126,90 TL + KDV
1.352,28 TL